Charts and Statistics: Useful information about clinical research before participating in a trial
The charts and statistics below help you learn more about clinical trials. All of this information is from trusted sources and can help you better understand clinical research and the experience of study volunteers.
If you have any questions about these charts and statistics, or would like to suggest useful information to include, please contact us at info@ciscrp.org
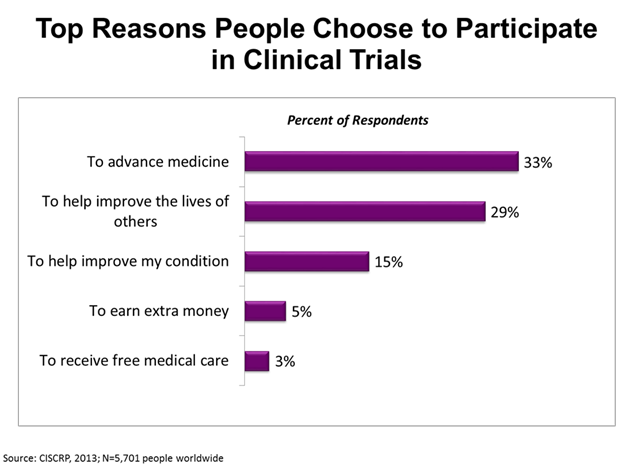
Each year millions of people chose to take part in clinical research and give the gift of their participation to help advance medical science. The chart above shows the top reasons why the people in a CISCRP survey choose to participate in clinical trials. The study volunteers in this global survey reported that the top two reasons they participated where to advance medicine (33%) and to help improve the lives of others (29%).
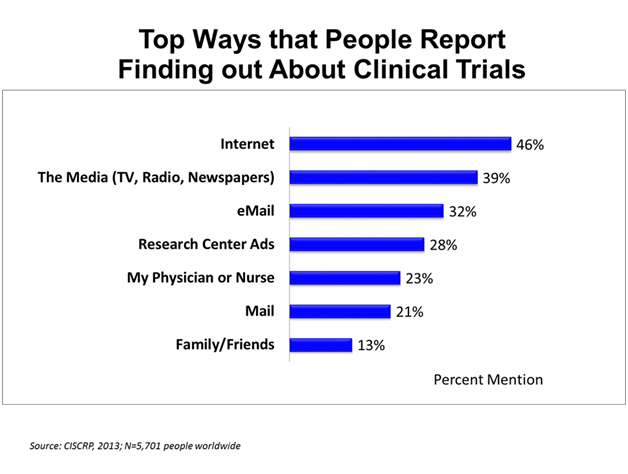
The chart above shows the results of a global CISCRP survey in which people were asked how they found out about clinical trials. Of the study volunteers in the survey, 46% reported that the top way they found out about clinical trials was the internet.
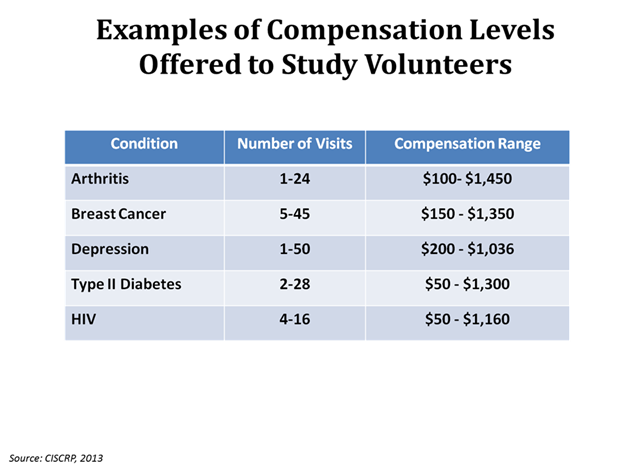
The chart above shows examples of the compensation offered to study volunteers for their participation in trials for five different medical conditions. The levels of compensation shown here change depending on the number of times a study participant must visit the study site.
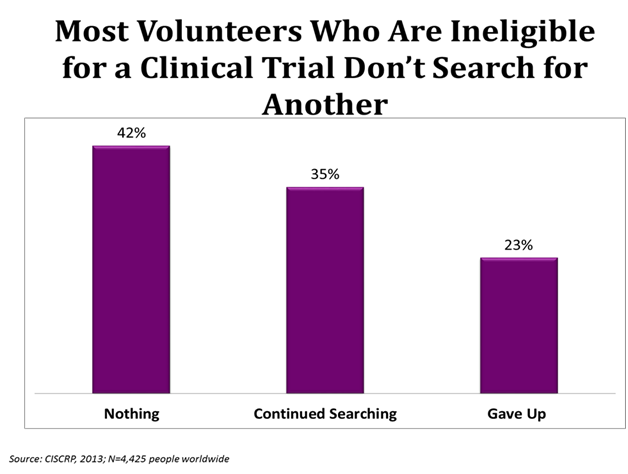
Sometimes people who are looking to join a clinical trial are not able to participate due to the restrictions of that specific study. In a worldwide survey conducted by CISCRP, 65% of people who were ineligable, or unable, to join a clinical trial reported that they either did “Nothing” or “Gave Up” searching for another trial.
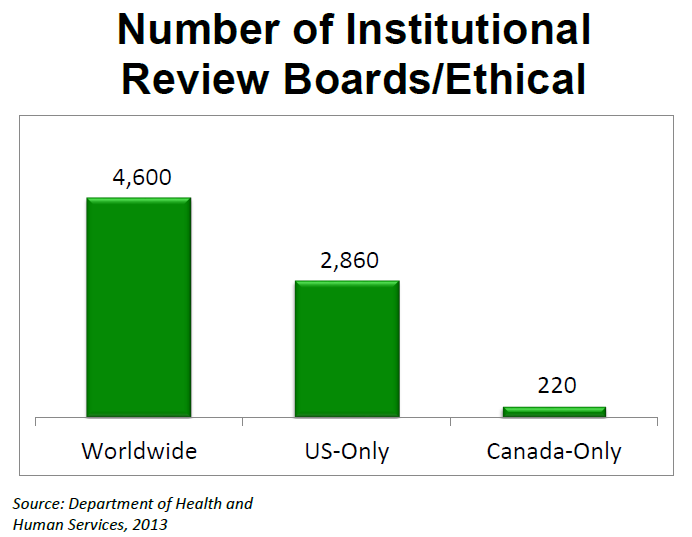
The chart above shows the number of Institutional Review Boards (IRBs)and/or Ethics Committees operating in the US, Canda, and Worldwide. In the US there are 2,860 IRBs that oversee research studies, including reviewing plans to conduct clinical trials and deciding whether or not the trial can start recruiting participants.