

Research Sponsor: Pfizer
Drug Studied: Toviaz®/ fesoterodine fumarate
National Clinical Trial #: NCT00611026
Protocol #: A0221046
Study Date: APRIL 4, 2008 to OCTOBER 20, 2009
Thank you!
As a clinical study volunteer, you belong to a large community of volunteers around the world. You help researchers answer important health questions and help them discover new medical treatments.
Thank you for participating in the clinical trial for fesoterodine fumarate, which took place between April 4, 2008 and October 20, 2009. Fesoterodine is also known by its brand name, Toviaz®. It is a prescription medicine used in adults to treat symptoms of a condition called overactive bladder.
Pfizer, the sponsor of this trial, thanks you for your help and thinks it is important for you to know the results of your trial. An independent non-profit called CISCRP prepared this summary of the trial results for you. Hopefully this summary helps you to understand and feel proud of your key role in medical research. If you have questions about the results, please speak with the doctor or staff at the trial site.
Click on the links below to find answers to questions about the study:
- What has happened since my trial ended?
- Why was the research needed?
- What kind of study was this?
- What happened during the study?
- What were the study results?
- What side effects did patients have?
- Where can I learn more about this clinical trial?
What has happened since my trial ended?
You were in the study for 14 weeks. However, the entire study took much longer. It took 18 months for the trial to finish. The trial had about 4,000 volunteers at 210 sites in 25 countries. The study was completed on October 20, 2009. When the study ended, the sponsor reviewed all the data and created a report of the results. This is a summary of that report.
Why was the research needed?
Researchers were looking for a better way to treat overactive bladder. People with overactive bladder have a strong need to urinate right away (which is called “urinary urgency”). They have to urinate too often (which is called “urinary frequency”). And some have leaking or wetting accidents due to a strong need to urinate (which is called “urge urinary incontinence”). Researchers wanted to learn how a medicine called fesoterodine fumarate treated these symptoms of overactive bladder.
To learn how fesoterodine treated overactive bladder, researchers compared it to another drug – tolterodine ER – and a placebo over a 12-week period. A placebo looks like a medicine but does not have any medicine in it. Researchers also wanted to study how safe fesoterodine was in this group of patients.
What kind of study was this?
This was a “blinded” study. In this trial, the patients and the researchers did not know who took which of the 3 treatments (fesoterodine, tolterodine ER, or placebo). Volunteers were picked for each treatment by chance alone. Both men and women took part in the study. They were all over 18 years old. They had overactive bladder problems and leaking accidents for at least 3 months before the study.
What happened during the study?
In this study, volunteers made 5 visits to the trial site. At first they were given a placebo to take for 2 weeks. Volunteers also kept a diary of their symptoms.
At the end of the 2 weeks, volunteers returned to the trial site. During this visit, volunteers were selected to stay in the study if their diaries showed that during the previous 3 days:
-
They had at least 1 leaking or wetting accident in 24 hours.
- And they had urinated at least 8 times a day.
Of the more than 4,000 volunteers screened, 2411 volunteers continued in the study and took a first dose. Each of these volunteers received 2 containers of medicine, a pack of white pills and a bottle of blue pills. They were told to take 1 white pill and 1 blue pill every day for 12 weeks.
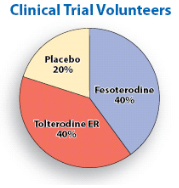
- 960 volunteers were in the fesoterodine group. They got a pack of white fesoterodine pills and a bottle of blue placebo pills.
- 973 volunteers were in the tolterodine ER group. They got a bottle of blue tolterodine ER pills and a pack of white placebo pills.
- 478 volunteers were in the placebo group. They got a pack of white placebo pills and a bottle of blue placebo pills.
Remember that there were 3 different types of treatments. Neither the researchers nor the study volunteers knew who had which treatment during the study.
The 2411 study volunteers went to the trial site 3 more times. These visits took place one week later (called the Week 1 visit), four weeks later (called the Week 4 visit) and 12 weeks later (called the Week 12 visit). At the Week 1 visit, the dose of medicine increased for study volunteers in the fesoterodine group. For 3 days before these visits, study volunteers wrote their symptoms in a diary. While at the visits, they also answered a survey about their symptoms and how overactive bladder affected their life.
The data gathered was used to prepare the full report of the trial results.
What were the study results?
The study suggests that 8 milligrams of fesoterodine treats symptoms of overactive bladder better than 4 milligrams of tolterodine ER or a placebo.
- Fesoterodine was better than tolterodine ER and the placebo in the following ways:
◦fewer leaking or wetting accidents per day
◦more urine passed each time volunteer urinated
◦fewer episodes of urinating per day – fewer sudden urges to urinate
- Fesoterodine was better than tolterodine ER starting from Week 4.
- Fesoterodine was more effective than the placebo but the same as tolterodine ER in the following ways:
◦increasing the amount of urine produced each time volunteer urinated
◦reducing the number of trips to the bathroom at night
What side effects did patients have?
The most common side effects in the study were dry mouth, constipation and headache. More volunteers who were treated with fesoterodine reported having these side effects than did volunteers treated with tolterodine ER or placebo.
Most side effects were mild or moderate.
The chart below shows the most common side effects that volunteers had while taking part in the study.
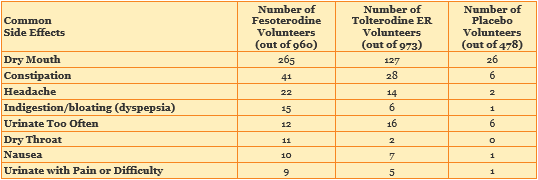
10 volunteers taking fesoterodine left the study because of dry mouth. 5 volunteers taking fesoterodine left the study because of pain or difficulty while urinating. These side effects were reported to be related to the treatment. 4 volunteers in the tolterodine ER group but none of the volunteers in the placebo group left the study because of dry mouth.
Some people had serious side effects: 7 people in the placebo group, 6 in the tolterodine ER group and 13 in the fesoterodine group had serious side effects. In the fesoterodine group, there were 2 serious side effects that were related to the treatment. The first was swelling related to kidney infection. The second was incomplete emptying of the bladder.
There were no deaths in the fesoterodine or tolterodine ER groups. There was 1 death in the placebo group, which was not related to the study drug
Where can I learn more about this clinical trial?
To listen to the summary of the trial results, call our toll free hotline at 1-877-538-7460.. If you have questions about the results, please speak with the doctor or staff at the trial site.
Again, thank you for volunteering. You have helped to answer an important question that could benefit public health.
Thank you
It is said that the greatest gift is one which is given anonymously, giving when you do not know whether you will get direct personal benefit.
This is the gift that you have given by taking part in a clinical trial. It is a brave and selfless act, one that advances medical knowledge and benefits public health. Thank you for the gift of your participation in clinical research.

The Center for Information & Study on Clinical Research Participation (CISCRP) is a non-profit organization focused on educating and informing the public about clinical research participation. CISCRP is not involved in recruiting patients for clinical trials, nor is it involved in conducting clinical trials.

