

Research Sponsor: Eli Lilly and Company
Research Collaborator: Genentech
Drug Studied: Gemcitabine (Gemzar®) plus Bevacizumab (Avastin®)
National Clinical Trial #: NCT00623233
Protocol #: B9E-US-S379
Study Date: March 2008 to January 2011
Full scientific report available at www.clinicaltrials.gov/show/results/NCT00623233
Thank you!
As a clinical study volunteer, you belong to a large community of volunteers around the world. You help researchers answer important health questions and help them discover new medical treatments.
Thank you for taking part in the clinical trial for two cancer drugs called gemcitabine (also called Gemzar®) and bevacizumab (also called Avastin®). This trial was started in March 2008 and finished in January 2011. You and 51 other women with advanced breast cancer helped researchers find out how long these two drugs might stop breast cancer from growing.
Eli Lilly and Company, the sponsor of this trial, thinks it is important for you to know the results. An independent non-profit called CISCRP prepared this summary of the results for you. The goal of this summary is to help you and your loved ones understand and feel proud of your key role in medical research. If you have questions about the results, please speak with the doctor or staff at your study site.
Click on the links below to find answers to questions about the study:
- Why was the research needed?
- What kind of study was this?
- What happened during the study?
- What were the study results?
- What side effects did patients have?
- Where can I learn more about this clinical trial?
What has happened since my trial ended?
When you left the study, other patients may have just been starting. The entire study took almost three years to complete, and included 52 women from 24 locations across the United States. Each patient stayed in the study until her cancer started getting worse or she had serious side effects. Unfortunately, since this was an advanced cancer, some patients passed away before the study ended.
Thanks to the generous spirit of all the patients, and of the loved ones who supported them through the study, researchers gained important information about the treatment of breast cancer. When the study came to an end in January, 2011, the sponsor began reviewing the data. Then the sponsor created a report of the results. This is a summary of that report.
Why was the research needed?
There are more than 2 million breast cancer survivors in the United States. However, breast cancer still takes the lives of as many as 40,000 women each year. Researchers are looking for medical treatments to help women with advanced breast cancer live longer and better lives.
One treatment that researchers thought might help is the combination of the drugs gemcitabine and bevacizumab. Gemcitabine is a chemotherapy drug used to treat breast cancer that has metastasized (spread to other parts of the body) and has not gotten better with other chemotherapy. Bevacizumab is a drug used to slow the growth of several types of cancer, most likely by decreasing blood supply to the tumor. The combination of gemcitabine and bevacizumab had previously been tested by patients with advanced cancer of the pancreas (a part of the digestive system).
In your clinical trial, patients helped find out how well treatment with gemcitabine plus bevacizumab works for women with a particular type of advanced breast cancer:
-
The cancer was “HER2-negative,” which means the tumor does not have a protein called HER2. This was important because different medical treatments would be used if the tumor had this protein.
-
The cancer had been treated in an early stage but it came back and your doctor knew that surgery could not cure it.
The main question your trial helped answer was how long the two drugs would stop this type of breast cancer from growing. Researchers also wanted to know if tumors would get smaller for any of the patients, and how many of the patients would survive for at least one year. All of the patients in this trial were expected to survive for at least 6 months. In addition, it was important to find out what side effects the two drugs might cause when used together.
What kind of study was this?
This was an “open-label study.” That means everyone—patients and study staff—knew which drugs you were getting. All of the patients took the same two drugs: gemcitabine plus bevacizumab. The patients were women who were at least 18 years old. Patients’ average age was 55 years.
What happened during the study?
Patients got the study drugs every 2 weeks. At your study site, staff gave you each drug as a liquid, through a needle inserted in one of your veins (an “IV infusion”). You got gemcitabine first. This took about 30 minutes. Then you got bevacizumab. This took about 90 minutes the first time. In later visits, study staff tried giving you bevacizumab over a shorter period of time, and checked that this was safe for you.
Study staff checked your health each time you went to your study site, including your blood pressure, temperature, heart rate, body weight, and how fast you were breathing. You also had blood tests and other lab work done. If you had tumors the staff could feel, these were measured every 4 weeks. Every 8 weeks, tumor size was measured with medical imaging equipment (CT scan, MRI scan, bone scan, or X-ray).
Patients stopped getting the study drugs if the cancer started to grow, or if different medical treatment was needed for other reasons. If the cancer grew, that meant it was getting worse. Patients also stopped getting the study drugs if the side effects were too bad, if their doctor thought stopping was in their best interest, or if they wanted to stop for any reason.
Patients kept going for follow-up visits after they stopped getting the study drugs. You came back to the study site 30 days after your last treatment. Again, study staff checked for cancer growth and side effects. After that, you had follow-up every two to three months until the study closed.
What were the study results?
In this study, 52 patients with HER2-negative advanced breast cancer took the study drugs gemcitabine and bevacizumab. Below is a summary of the medical questions that were asked in the study, and what the study results were.
For how long did the cancer stop growing?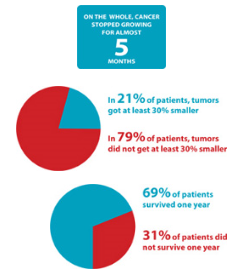
On the whole, patients’ cancer stopped growing for almost 5 months. But the results were different for each patient.
In how many patients did tumors get at least 30% smaller?
Measured tumors got at least 30% smaller in 21% of patients. In the other 79% of patients, tumors either grew larger, stayed the same size, or got smaller but by less than 30%.
How many patients survived their cancer for at least 1 year after starting the study drugs?
Two thirds of the patients (69%) survived their breast cancer for at least one year after starting the study drugs. One third of the patients (31%) passed away within one year.
What side effects did patients have??
A side effect is any medical problem from a drug or treatment. A lot of research is needed to know whether a medical problem is caused by a drug or treatment. So, when new drugs are being studied, researchers keep track of all medical problems that patients have. These medical problems are called “adverse events,” and may or may not be caused by the study drugs. A list of adverse events is an important part of the study results. This section tells you about the adverse events found in your study.
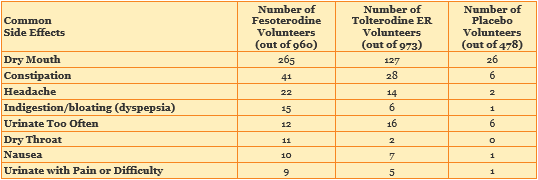
51 of 52 patients had less-serious adverse events. These adverse events were not life-threatening. The table below shows the most common of the fifty types of less-serious adverse events found in this study.
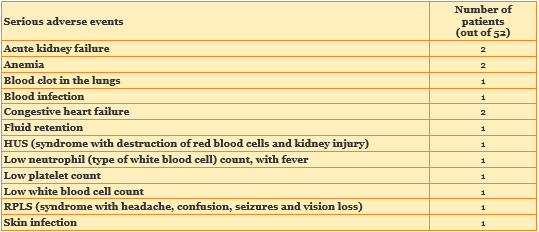
18 of 52 patients had adverse events considered serious, and 34 of 52 did not. An adverse event is considered serious if it is life-threatening, if it puts you in the hospital or keeps you there for a long time, if it causes a birth defect, or if it could cause long-term disability.
The table below shows twelve of the forty-four types of serious adverse events that patients in this study had. Each type of serious adverse event was experienced by 1 or 2 patients.
Full list can be found on the U.S. government’s clinical trial website
at www.clinicaltrials.gov/show/results/NCT00623233
Where can I learn more about this clinical trial?
The full scientific report can be found on the US government’s clinical trial website at www.clinicaltrials.gov/show/results/NCT00623233. If you have questions about the results, please speak with the doctor or staff at your study site.
Thank you!
It is said that the greatest gift is one which is given anonymously, giving when you do not know whether you will get direct personal benefit.
This is the gift that you have given by taking part in a clinical trial. It is a brave and selfless act, one that advances medical knowledge and benefits public health. Thank you for the gift of your participation in clinical research.

The Center for Information & Study on Clinical Research Participation (CISCRP) is a non-profit organization focused on educating and informing the public about clinical research participation. CISCRP is not involved in recruiting patients for clinical trials, nor is it involved in conducting clinical trials.

