Research Sponsor: Eli Lilly and Company
Drug Studied: Duloxetine (Cymbalta®)
National Clinical Trial #: NCT01018680
Protocol #: F1J-US-HMGL
Study Date: November 2009 to April 2011
Thank you!
As a clinical study volunteer, you belong to a large community of volunteers around the world. You help researchers answer important health questions and help them discover new medical treatments.
Thank you for taking part in the clinical study for the drug duloxetine, which took place between November 2009 and April 2011. Duloxetine is also known by its brand name, Cymbalta®. It is a prescription drug used in adults to treat the pain of osteoarthritis, a common disease of the joints.
Eli Lilly and Company, the sponsor of this trial, thinks it is important for you to know the results. An independent non-profit called CISCRP prepared this summary of the results for you. We hope it helps you understand and feel proud of your key role in medical research. If you have questions about the results, please speak with the doctor or staff at your study site.
Click on the links below to find answers to questions about the study:
- What has happened since my trial ended?
- Why was the research needed?
- What kind of study was this?
- What happened during the study?
- What were the study results?
- What side effects did patients have?
- Where can I learn more about this clinical trial?
What has happened since my trial ended?
You were in the study for up to 14 weeks, but the entire study took much longer. It took 17 months for the study to finish. The study had 524 patients at 35 locations across the United States. When the study ended in April, 2011, the sponsor began reviewing the data. Then the sponsor created a report of the results. This is a summary of that report.
Why was the research needed?
Researchers were looking for a better way to treat knee pain in people with osteoarthritis. This pain is usually treated with drugs called “NSAIDs” (Non-Steroidal Anti-Inflammatory Drugs), such as aspirin and ibuprofen. But some people who take NSAIDs for osteoarthritis still have pain. Researchers wanted to find out if a drug called duloxetine would help people who take NSAIDs, but still have pain.
Previous studies showed that duloxetine can decrease osteoarthritis knee pain and increase knee function. Now researchers wanted to know:
- Does duloxetine help patients with osteoarthritis knee pain who are taking NSAIDs, but still have pain?
- Is it safe to take duloxetine and NSAIDs at the same time?
To answer these questions, researchers asked for the help of men and women like you. All of the patients in your study were over 40 years old, had osteoarthritis in their knee, and were taking NSAIDs but still had knee pain.
What kind of study was this?
This study compared two groups of patients to find out if duloxetine was safe and relieved pain for patients already taking NSAIDs. Researchers used a computer program to randomly put patients into the two groups:
- Duloxetine Group: 264 patients took NSAIDs and duloxetine.
- Placebo Group: 260 patients took NSAIDs and a placebo.
A “placebo” looks like medicine, but does not have any medicine in it. Using a placebo helps make study results fair and unbiased, because researchers and patients cannot tell who is taking a placebo, and who is taking the real medicine.
What happened during the study?
In the first 2 weeks, doctors helped patients find the best dose of NSAIDs. Patients also started taking a drug called omeprazole every day to help with the stomach pain that NSAIDs can cause.
Over the next 10 weeks, patients were treated with either duloxetine and NSAIDs, or with a placebo and NSAIDs. Each duloxetine pill had 60 milligrams of the drug. The placebo pills looked like the duloxetine pills, but had no medicine in them. At first, all patients took 1 pill every day. Later, patients who still had a lot of knee pain took 2 pills every day. Patients saw their study doctor 5 times during the treatment period. During those visits, patients completed surveys that rated how much their knee pain interfered with their lives. Patients also used a telephone system to keep a daily diary of their pain, rating it from 0 (no pain) to 10 (extreme pain). Many of the study results were measured when patients had been treated for 8 weeks.
The study ended with a 2-week period, during which patients slowly stopped taking the study treatment with the doctor’s help.
What were the study results?
In this study, patients taking duloxetine with NSAIDs (Duloxetine Group) had less knee pain and had an easier time with daily activities than patients taking the placebo with NSAIDs (Placebo Group). Below is a summary of the medical questions that were asked in this study, and the study results.
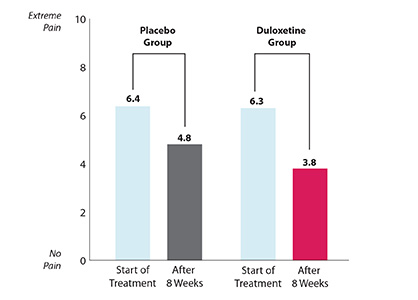
| Did duloxetine with NSAIDs help relieve osteoarthritis knee pain? Yes. After 8 weeks of treatment, both groups of patients still had some pain, but less than when they started treatment. Patients who took duloxetine with NSAIDs were helped more than patients who took the placebo with NSAIDs.
AVERAGE OSTEOARTHRITIS KNEE PAIN
|
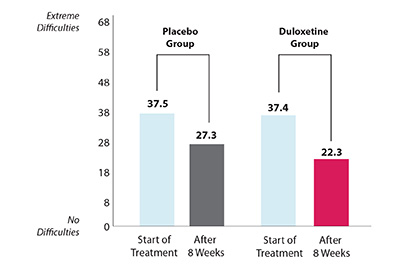
| Did duloxetine make daily activities easier? Yes. After 8 weeks of treatment, both groups of patients had less difficulty with daily activities such as walking, putting on socks, and doing chores. Patients who took duloxetine with NSAIDs were helped more than patients who took the placebo with NSAIDs.
AVERAGE DIFFICULTY OF DAILY ACTIVITIES
|
Did duloxetine help in other ways?
- After 8 weeks of treatment, both groups of patients said they felt better. Patients also said they had less trouble sleeping, working, having good relationships, and enjoying life, because they had less pain. Patients who took duloxetine with NSAIDs were helped more than patients who took the placebo with NSAIDs.
- Patients taking duloxetine with NSAIDs had the same amount of depression, tiredness, and worry as patients taking placebo with NSAIDs.
What side effects did patients have??
A side effect is any medical problem caused by a drug or treatment. A lot of research is needed to know whether a drug causes a medical problem. So, when new drugs are being studied, researchers keep track of all medical problems that patients have. These medical problems are called “adverse events,” and may or may not be caused by the study drugs.
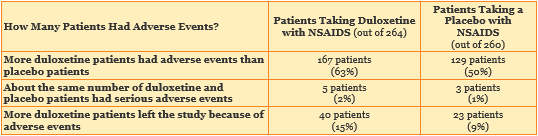
Did more patients taking duloxetine with NSAIDs have adverse events?
Yes. Half (50%) of the Placebo Group patients and more than half (63%) of the Duloxetine Group patients had adverse events. This suggests that some adverse events were caused by duloxetine. Most adverse events in this study were not serious. The table below shows how many patients had adverse events.
What serious adverse events did patients have?
An adverse event is considered “serious” when it is life-threatening, causes lasting problems, or needs hospital care. In the Placebo Group, the following serious adverse events each happened to 1 patient: breast cancer, gallbladder disease, pancreatitis, stroke. In the Duloxetine Group, the following serious adverse events each happened to 1 patient: coronary artery disease, fainting, fall, serious infection from the urinary tract, and spread of cancer to the nervous system.
What were the most common adverse events in this study?
The most common adverse events were nausea, dry mouth, and constipation. None of these adverse events was serious, but each happened more often to patients taking duloxetine with NSAIDs than to patients taking placebo with NSAIDs. The table below shows the most common of the 61 types of adverse events found in this study.

Full list can be found on the U.S. government’s clinical trial website
at www.clinicaltrials.gov/show/results/NCT01018680
Where can I learn more about this clinical trial?
The full scientific report can be found on the US government’s clinical trial website at www.clinicaltrials.gov/show/results/NCT01018680. If you have questions about the results, please speak with the doctor or staff at your study site.
Thank you
It is said that the greatest gift is one which is given anonymously, giving when you do not know whether you will get direct personal benefit.
This is the gift that you have given by taking part in a clinical trial. It is a brave and selfless act, one that advances medical knowledge and benefits public health. Thank you for the gift of your participation in clinical research.

The Center for Information & Study on Clinical Research Participation (CISCRP) is a non-profit organization focused on educating and informing the public about clinical research participation. CISCRP is not involved in recruiting patients for clinical trials, nor is it involved in conducting clinical trials.