

Research Sponsor: Pfizer
Drug Studied: Pregabalin (Lyrica®)
National Clinical Trial #: NCT01057693
Protocol #: A0081242
Study Date: March 2010 to January 2012
Thank you!
As a clinical study volunteer, you belong to a large community of volunteers around the world. You help researchers answer important health questions and help them discover new medical treatments.
Thank you for participating in the clinical trial for the drug pregabalin, which took place between March 2010 and January 2012. Pregabalin is also known by its brand name, Lyrica®. It is a prescription medicine used in adults to treat the pain of damaged nerves in their arms, hands, legs or feet, caused by diabetes.
Pfizer, the sponsor of this trial, thanks you for your help and thinks it is important for you to know the results of your trial. An independent non-profit called CISCRP prepared this summary of the trial results for you. We hope it helps you to understand and feel proud of your key role in medical research. If you have questions about the results, please speak with the doctor or staff at your trial site.
Click on the links below to find answers to questions about the study:
- What has happened since my trial ended?
- Why was the research needed?
- What kind of study was this?
- What happened during the study?
- What were the study results?
- What side effects did patients have?
- Where can I learn more about this clinical trial?
What has happened since my trial ended?
When you left the study, other patients may have just been starting. The entire study took almost 2 years to finish, and included 665 volunteers at 129 locations in the US, Canada, and South Africa. When the study ended in January 2012, the sponsor reviewed all the data and created a report of the results. This is a summary of that report.
Why was the research needed?
Diabetes can cause painful damage to the nerves in the arms, hands, legs, and feet. This is called “diabetic peripheral neuropathy” or DPN. Some treatments for DPN do not relieve pain for everyone, and sometimes treatments stop working after awhile.
Researchers wanted to know how well and for how long pregabalin treated the pain of DPN in a group of patients who were taking medicine for DPN, but still had pain. They also wanted to find out how safe pregabalin was in this group of patients.
To answer these questions, researchers asked for the help of men and women like you. All of the patients in your study were over 18 years old and had moderate to severe DPN pain.
What kind of study was this?
This study compared pregabalin with placebo for the treatment of DPN. A “placebo” looks like a medicine but does not have any medicine in it. Comparing pregabalin to placebo helps researchers understand how well pregabalin works, and how safe it is.
This study was done in 2 phases or parts: first a single-blind phase, and then a double-blind phase. “Single-blind” means that only the researchers knew what treatment the patient took. “Double-blind” means that neither the researchers nor the patients knew which treatment the patient took.
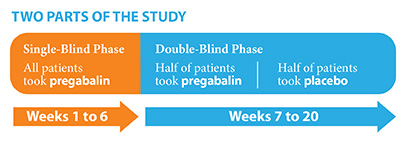
In the single-blind phase, all patients took pregabalin for 6 weeks. In the double-blind phase, half of the patients took pregabalin, and half took placebo. This phase lasted for up to 14 weeks.
What happened during the study?
Single-blind Phase:
During this phase, patients stopped taking the pain medicine their doctor prescribed, and started taking 150 mg of pregabalin every day. Doctors asked patients to keep a pain diary and rate their pain from 0 (no pain) to 10 (extreme pain) every day. If their pain did not get better, doctors could give patients up to 300 mg of pregabalin every day.
If a patient’s pain was not at least 30% lower on the pain scale after 6 weeks, the patient stopped taking pregabalin with the help of the doctor and left the study.
Double-blind Phase:
In this phase, half the patients took pregabalin, and the other half took the placebo. All patients took 1 capsule 3 times each day. Patients kept a pain diary and a sleep diary, and the doctor reviewed these during each clinic visit. Patients also told doctors how they felt, and took surveys about their pain, sleep, and mood. During three clinic visits, patients had blood drawn for testing.
The data gathered from both phases of the study was used to prepare the full report of the trial results.
What were the study results?
 Did pregabalin help relieve the pain of DPN?
Did pregabalin help relieve the pain of DPN?
No, pregabalin did not relieve the pain of DPN any better than the placebo, which contained no medicine. Let’s take a closer look at the study results to see what happened in each phase of the study.
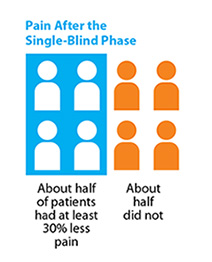
Single-blind Phase:
In this part of the study, 665 patients took pregabalin for up to six weeks. At the end, about half of the patients had at least 30% less pain and could move on to the double-blind phase, and half did not.
Double-blind Phase:
There were 294 patients in this part of the study: 147 patients took pregabalin, and 147 took the placebo. Two more patients were assigned to the placebo group, but decided to leave the study before treatment started. The chart below compares how much pain both groups of patients had at the start and end of the study.
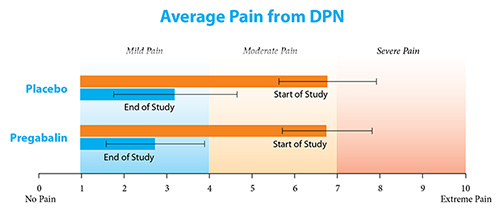
All patients started the study with a similar amount of pain, on average. At the end of the study, patients who took pregabalin in the double blind phase had about the same amount of pain as patients who took placebo in the double blind phase, on average. This means that pregabalin did not work better than the placebo, which had no medicine in it.
Did pregabalin help in other ways?
Most patients who finished the study felt better than when they started. Patients had less trouble sleeping, and less anxiety and depression. Patients also said their daily activities were easier to complete, and moving was easier.
However, in the double-blind phase, patients who took pregabalin were not helped any more than patients who took the placebo, which contained no medicine.
What side effects did patients have?
A side effect is any medical problem caused by a drug or treatment. A lot of research is needed to know whether a drug causes a medical problem. So, when new drugs are being studied, researchers keep track of all medical problems that patients have.
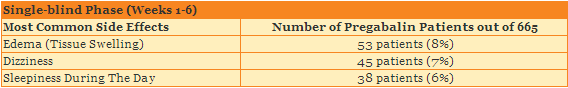
What side effects did patients have in the single-blind phase?
In the single-blinded phase of the study, 331 out of 665 patients (50%) had a side effect after taking pregabalin, and 45 patients (7%) left the study because of a side effect. The table below shows the most common side effects in the single-blind phase.
What side effects did patients have in the double-blind phase?
In the pregabalin group, 86 out of 147 patients (58%) had side effects, and 8 patients left the study because of a side effect. In the placebo group, 94 out of 149 patients (63%) had side effects, and 11 patients left the study because of a side effect.
The table below shows the most common side effects that patients had in the pregabalin and placebo groups. When similar numbers of patients in each group have a side effect, it means the side effect probably was not caused by the medicine.
Did any patients have serious side effects?
A side effect is considered “serious” when it is life-threatening, causes lasting problems, or needs hospital care. Some patients in the study had serious side effects, but no patients died during the study.
In the single-blind phase, 17 out of 665 patients (3%) had serious side effects. In the double-blind phase, 11 out of 147 patients who took pregabalin (7%) had serious side effects. And 6 out of 149 patients who took the placebo (4%) had serious side effects.
A full list of side effects in this study can be found on the U.S. Government’s clinical trial website at http://clinicaltrials.gov/ct2/show/results/NCT01057693.
Where can I learn more about this clinical trial?
Please remember that researchers look at the results of many studies to find out which medicines work best and are safest for patients. If you have questions about the results, please speak with the doctor or staff at your study site.
To listen to the summary of the clinical trial results, please call our toll free hotline at 1-888-995-5132. To view and download this summary of the clinical trial results online in print format (PDF) , please click here.
Again, thank you for volunteering. You have helped to answer an important question that could benefit public health.
Thank you
It is said that the greatest gift is one which is given anonymously, giving when you do not know whether you will get direct personal benefit.
This is the gift that you have given by taking part in a clinical trial. It is a brave and selfless act, one that advances medical knowledge and benefits public health. Thank you for the gift of your participation in clinical research.

The Center for Information & Study on Clinical Research Participation (CISCRP) is a non-profit organization focused on educating and informing the public about clinical research participation. CISCRP is not involved in recruiting patients for clinical trials, nor is it involved in conducting clinical trials.


