Charts and Statistics: Useful information about clinical research after participating in a trial
The charts and statistics below help you learn more about clinical trials. All of this information is from trusted sources and can help you better understand clinical research and the experience of study volunteers.
If you have any questions about these charts and statistics, or would like to suggest useful information to include, please contact us at info@ciscrp.org
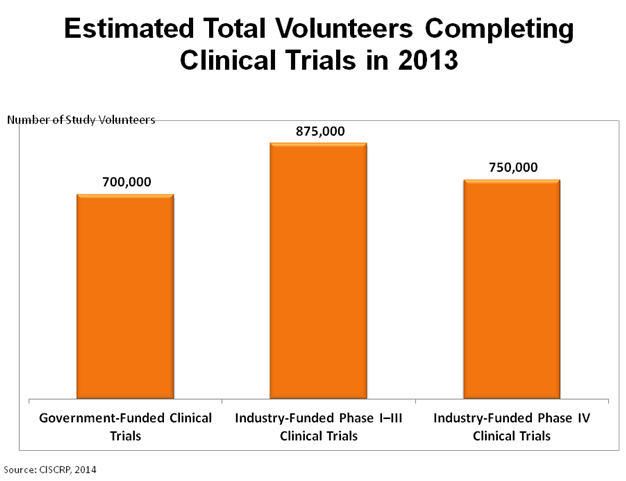
Clinical trial volunteers play a very important role in the process of developing safe and useful medical treatments. The vertical bars in the chart above show the number of people estimated to finish their research study in three different types of trials in 2013. By these estimates, over 2.2 million people will have completed their participation in clinical trials in 2013.
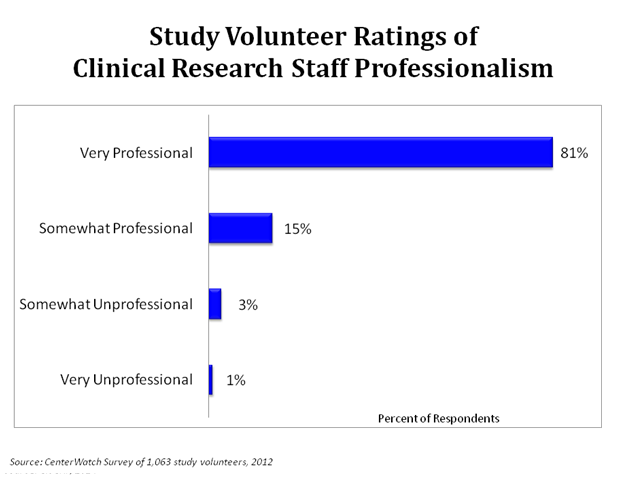
In a survey of over 1,000 study volunteers, 96% thought the study staff were “Very Professional” or “Somewhat Professional”.
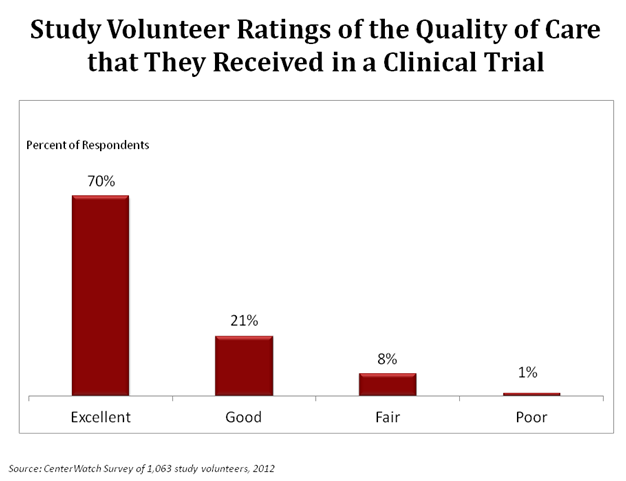
In a survey of over 1,000 study volunteers, 91% thought the quality of care they received in their clinical trial was “Excellent” or “Good”. About 8% of these volunteers thought the quality of care they received was “Fair”.
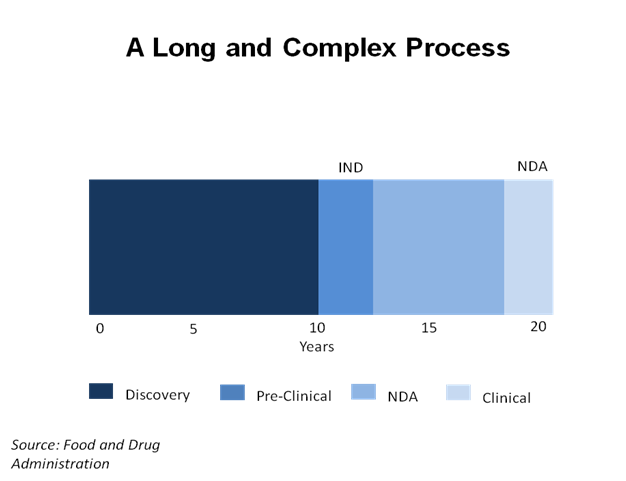
The process of creating a safe and useful medicine can take a very long time. The chart above shows that it can take over 15 years before a new drug is used in clinical trials. The clinical trial stage of development may only take a few years, but it is a very important part of the overall process, which can take 20 years from start to finish.
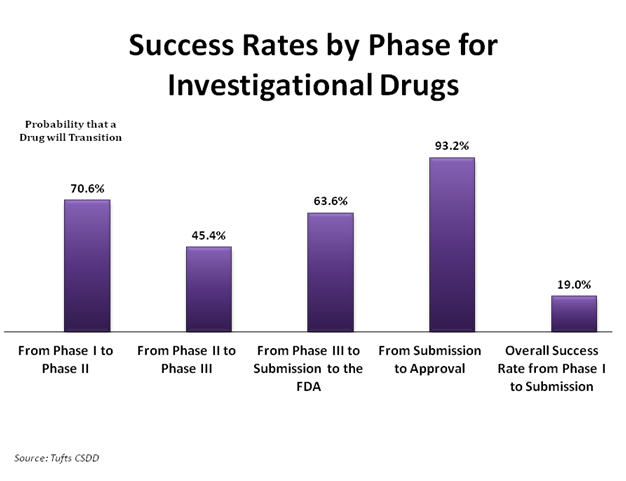
The development of a drug can take a very long time, and sometimes even when the drug reaches the clinical trial stage, it does not become an approved treatment. The chart above shows that about 1 out of every 5 drugs in the clinical trial process are submitted to the Food and Drug Administration (FDA) for approval. However, of the drugs that are submitted for approval, there is a 93.2% chance it will be approved.